4. Theoretical Consideration (1) -- Rust Circuit
Some considerations are discussed below to examine the anticorrosive effect
of S-wave. First, look at the mechanism in which iron corrosion and rust
are produced repeatedly.
Corrosion is a process in which the performance of a metal changes or wears
off as a result of chemical or electrochemical reaction between the metal
and the environment. Anti-corrosion means protection of a metal from corrosion,
while anti-rust means anti-corrosion in relation to iron or steel.
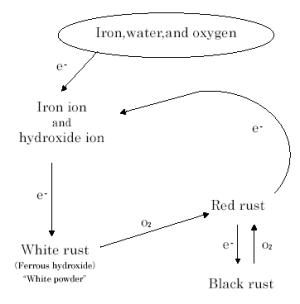 |
Figure 4 Rust Circuit Diagram
("white rust" is the coined word by the author) |
Necessities for the corrosion of ion in water are: there is dissolved oxygen,
and galvanic action occurs (iron oxidation and oxygen reduction occur electrically
equivalently and simultaneously).
There also are microbiologically-induced corrosion phenomena.
For iron rust that progresses repeatedly, reaction formulas are as follows
(Figure 4):
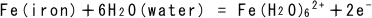 (1)
(1)
When an iron atom dissolves into water as it is ionized, it releases two
electrons. This is an oxidation process. If a single electron is released,
the iron tends to stay away from getting ionized, so it does not take the
first step to rusting.
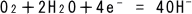 (2)
(2)
Dissolved oxygen in water reacts with water molecules and electrons e-
(from Eq. (1)) to form a hydroxide ion. Note that oxidation-reduction reactions
in Eq. (1) and (2), respectively, occur simultaneously.
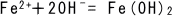 (3)
(3)
The dissolved iron ion 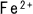 is reduced through reaction with
is reduced through reaction with 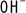 to produce ferrous hydroxide.
to produce ferrous hydroxide.
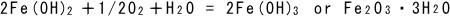 (4)
(4)
The ferrous hydroxide is immediately oxidized to form ferric hydroxide
in red. As dehydration progresses, the ferrous hydroxide forms Fe2O3EH2O or FeOOH (hydrated iron oxide).
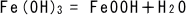 (5)
(5)
The ferric hydroxide turns to red rust (hydrated iron oxide).
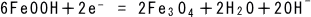 (6)
(6)
The red rust FeOOH reacts with the two electrons dissolved in Eq. (1) to
produce black rust Fe3O4 (triiron tetraoxide) and hydroxide ions. The hydroxide ions work in the
reductive reaction of Eq. (3) to convert the iron ion to ferrous hydroxide.
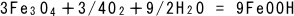 (7)
(7)
The back rust is oxidized to form red rust.
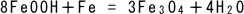 (8)
(8)
When the red rust reacts with the iron in the substrata and is reduced
by iron ions, a relatively hard black rust (magnetite type) is formed.
This black result is hard and stable as far as oxygen is deficient (covered
by red rust), but once it reacts with oxygen, it turns to the red rust
as in Eq. (7).
As seen above, rust oxides grow by repeating oxidation and reduction, like
creatures breathe in and out. Unlike an oxidation protective film of dense
structure over a copper substrate, the loose-structured rusts continue
growing. The energy that causes iron oxidation in pipes thousands of meters
long amounts to a considerably large quantity of negative power. Positive
energy in excess of that quantity must be accurately kept supplied to stop
the corrosion progress.
With no energy supplied from outside, every phenomenon in nature progresses
towards a loss of energy, that is, from solid to liquid to gas, where molecular
and atomic arrangement is disordered and loosened. With no energy supplied
from outside, most materials and creatures progresses towards oxidation
reaction (deprived of electrons and hydrogen, and coupled with oxygen).
Iron is refined industrially by reducing the stable iron oxides existing
in the earth. Then, it rusts, back to iron oxide, and returns to the earth.
The color of earth is a mixture of colors of iron oxides and organic substances.
Anti-corrosion (anti-rust) is a matter of how oxidation reaction can be
slowed down by artificial means. Iron will rust. Preventing rusting by
means of rust is the enzymatic technology that uses a minimum energy of
S-wave.
5. Theoretical Consideration (2) -- What is Excitation?
S-wave excites water molecules, as well as iron, copper, and silica/calcium.
Excitation of water molecules means transition to high-energy state, and
its vibration breaks the intermolecular force of aggregates of large molecules
into smaller aggregates.
Water molecule groups, which are mesoscopic (a being between macro-state
or micro-state), are believed to have special activity and properties that
have yet to be elucidated. To excite iron or copper is to raise its energy
level, or in other words, to build up energy, When silica or calcium is
excited, for example, the crystal germ is vibrated and the resultant temperature
change suppresses precipitation, while scales already in crystallized state
have their crystal germs vibrated and the binding power dissipated, leading
to descaling.
Where S-wave propagates in standing water, an aggregate of water molecules
like an iceberg is broken into pieces like small pearl balls, which also
are excited, allowing the S-wave to propagate in water through long pipes
with little resistance as if skating over ice. When S-wave is generated
in a high-elevation water tank, excitation of water molecule groups produces
energy, so that the S-wave is considered to move as a resonant wave in
a thermodynamically identical direction until it reaches the faucet in
the same water feed pipe on the first floor.
6. Theoretical Consideration (3) -- Iron and Copper in Oxygen
In humans, various enzymes dissolve bad active oxygen species to protect
the body. Acting on the most important part of enzymes are metal complexes
such as iron and copper. Generally, biometallic elements are called metalloenzymes
or metalloproteins.
Intraerythrocyte hemoglobins in vertebrates have bivalent iron, and if
oxygen exists in large quantity, combine with oxygen and turn red. If a
plenty of carbon dioxide exists, the hemoglobins release oxygen, and instead,
combine with carbon dioxide and turn dark red. The electron transfer system
in the endomembrane of mitochondria in cells converts oxygen to energy
through a chain reaction of reduced bivalent iron ion (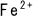 ) and oxidized trivalent iron ion (
) and oxidized trivalent iron ion (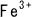 ).
).
In molluscs and crustaceans, oxygen is carried on the center of hemocyanin
(a deep blue, copper-containing pigment).
The reason why iron plays a particularly important part among other biometallic
elements is said to lie in the development of life's evolution and the
characteristics of iron. In the primordial period of earth, large amounts
of iron were dissolved in the sea, no oxygen was in the sky, and seaweed
perform photosynthesis. In such an environment, iron can obtain the largest
range of oxidation-reduction potential through binding with complexes,
making it suitable for various vital activities.
Oxidation-reduction potentials unavailable with iron were supposedly obtained
via combination of copper and complexes. When iron is activated, aerobic
microorganisms are always activated. This means that raised energy of enzymes
provides rejuvenation. The more energy charged, the greater resistance
to bad active oxygen.
7. Theoretical Consideration (4) -- Soil and Rust in Rice Paddies
As iron is formed and processed, the surfaces of pipes has several elements
condensed, such as carbon, manganese, silicon, chromium, aluminum, and
phosphor. Where there is rust, there are organic compounds and microorganisms.
Actual constituents of rust are iron, alloy elements, and organic compounds.
Rust resembles paddy soil having a large iron content. The oxidation-reduction
potential of paddy soil is approximately -0.2 to +0.3 volts. A higher value
of this potential means a more oxidized state of soil, and a lower value
means a more reduced state. The border between oxidized state and reduced
state is +0.3 volts. Oxidation-reduction potentials are -0.5 to +0.3 volts
for iron, and approximately +0.4 volts for copper. Iron in paddy soil is
ferric (trivalent) when contained in oxidized environment (e.g., surface
soil); it is ferrous (bivalent) when in reduced environment (e.g., plowed
soil). Depending on whether oxygen is in existence (in large quantity)
or nonexistence (in small amount), iron changes reversibly just as it does
in vivi.
In steel pipes, however, corrosion is irreversible, or a one-way process.
This difference in behavior is due to the energy imparted to iron. Black
rust (Fe3O4) contains a ferrous iron molecule and two ferric iron molecules per four
oxygen molecules. It is strongly magnetic and somewhat conductive. This
implies that the electrons of the ferrous molecule are freely moving around.
When an electron reaches oxygen, the black rust immediately turns to red
rust. Metaphorically speaking, ferrous iron (which has a higher energy
level than ferric iron) becomes tired and let go hold of an electron, to
find itself to be a ferric iron. However, what happens if we excite iron
to continue supplying positive energy. It could turn to ferrous iron.


| Copyright (c)1995- ASAHI INC. All Rights Reserved. |
(1)
(2)
(3)
is reduced through reaction with
to produce ferrous hydroxide.
(4)
(5)
(6)
(7)
(8)
) and oxidized trivalent iron ion (
).